Research Summary
Our fundamental question:
Why are eukaryotic organisms – from a single cell to an entire organism – organized into compartments, and how does this compartmentalization shape metabolism in health and disease?
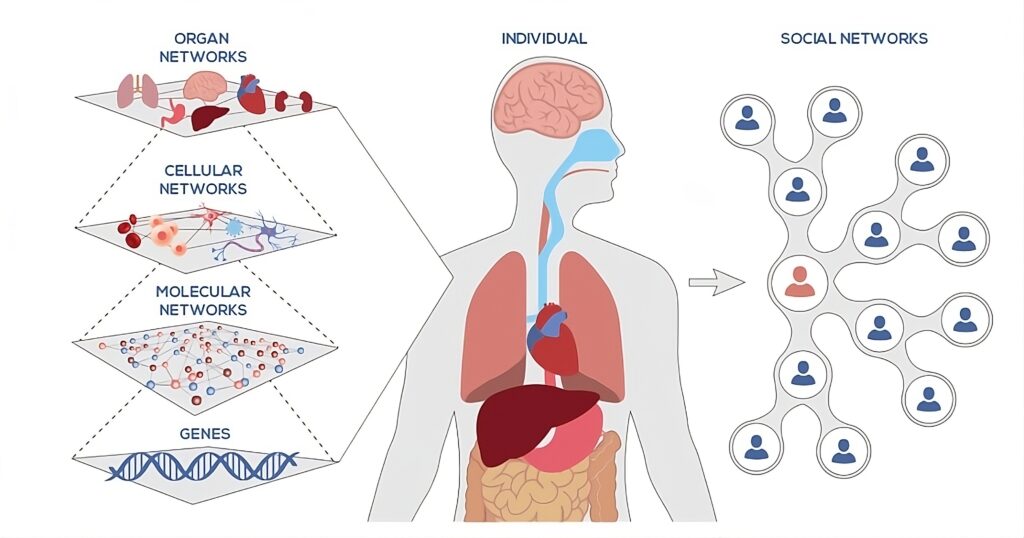
The Won Lee Lab develops and applies fluxomics approaches to uncover how metabolism operates across scales – from subcellular compartments to whole organisms. We pioneered subcellular fluxomics by performing stable isotope tracing in intact cells followed by rapid organelle isolation, metabolomics, and computational modeling, enabling the quantification of compartment-specific fluxes. We later established methods for interorgan and organismal fluxomics, conducting tracer studies in freely moving live animals to map how metabolites flow across tissues and circulation. Having uniquely developed and mastered both of these approaches, our lab is among the very few capable of integrating metabolism seamlessly from the subcellular to the whole-organism level.
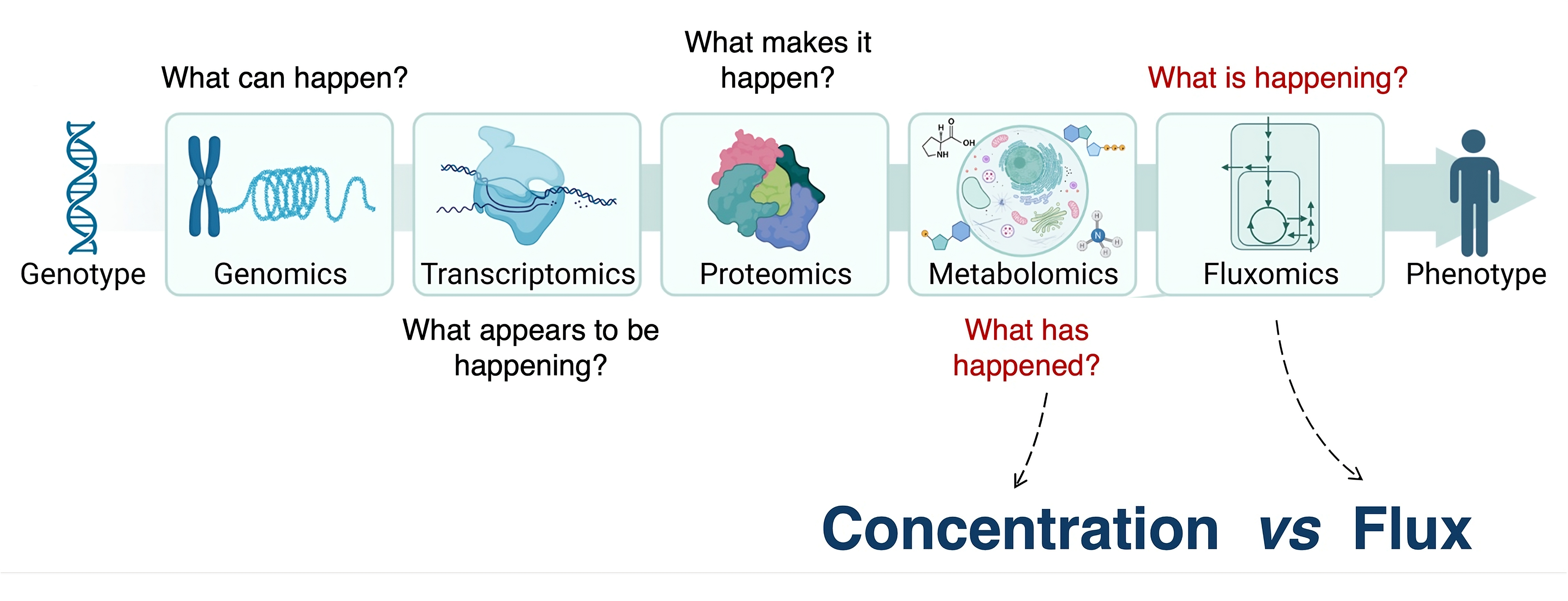
A central theme of our work is moving beyond simply cataloging what and how many metabolites are present (spatial metabolomics) toward quantifying what is actively happening in each compartment (spatial fluxomics). By combining stable isotope tracing with spatially resolved analysis, we aim to measure reaction rates in their native subcellular, tissue, and organismal contexts. This framework allows us to ask not just where metabolites are, but how they are used, coordinated, and reprogrammed in health and disease.
Research Projects
Develop next generation methods for subcellular fluxomics
Building the fastest, cleanest way to see metabolism inside real compartments
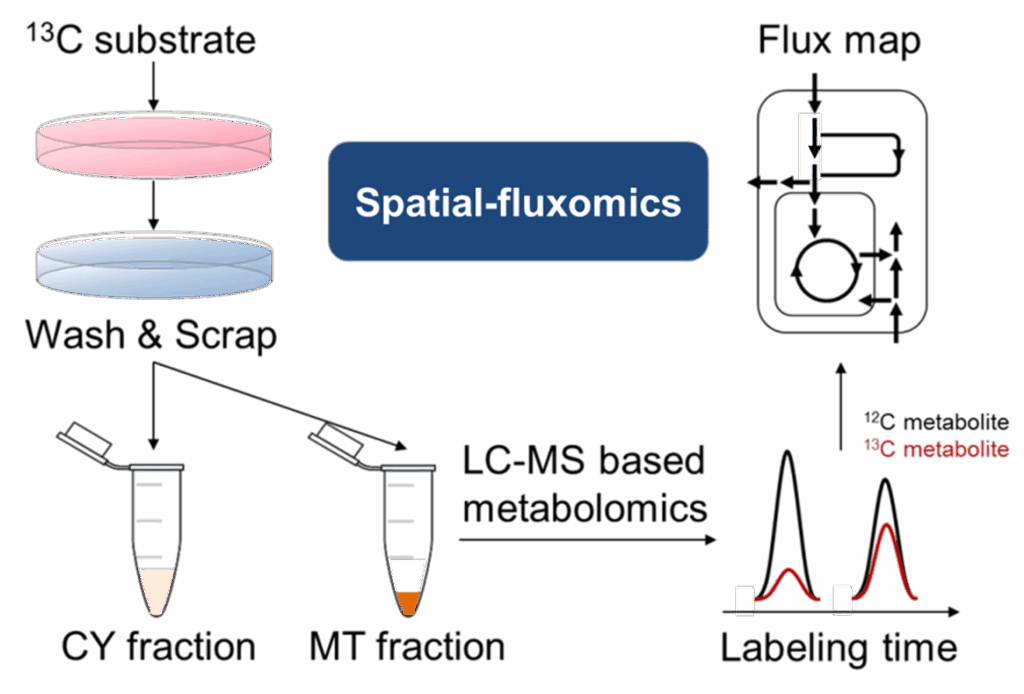
Metabolism doesn’t happen uniformly inside a cell – it’s compartmentalized. We have shown that mitochondria, cytosol, and other organelles each run distinct reactions, and their cross-talk often determines whether a cell grows, adapts, or dies (1, 2). Yet our ability to measure metabolism at this level is limited: existing fractionation methods are slow, prone to contamination, and often blur together signals from different compartments. To fully understand how metabolic pathways are wired, we need methods that can deliver rapid, pure, and reproducible subcellular fractions, ideally within a minute, in both cultured cells and tissues. Our lab is developing new tools to meet this need, enabling the first flux-level view of metabolism in real cellular compartments in both in vitro and in vivo, and opening the door to discoveries that bulk analyses have long concealed.
Mitochondrial control of in situ and in vivo (patho)physiology
How mitochondria gate whole-body (patho)physiology.
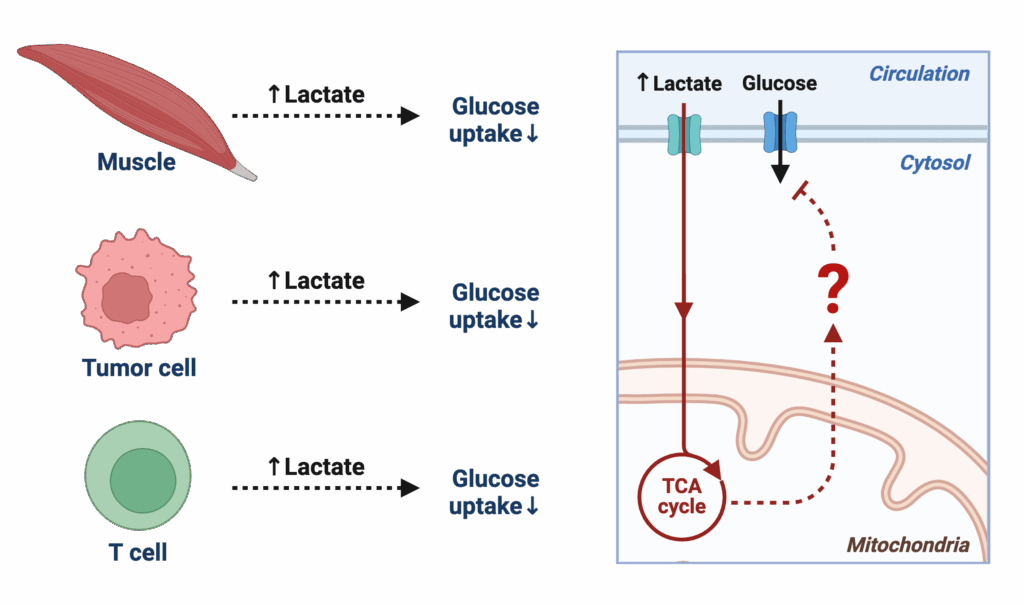
Mitochondria are central hubs that decide whether nutrients are burned for energy, stored, or repurposed for signaling. Our recent work (3) showed that elevated lactate rapidly induces insulin resistance in skeletal muscle and the whole body, revealing that mitochondria don’t just make ATP – they act as gatekeepers of systemic physiology. Yet the molecular logic by which mitochondria sense and respond to their environment in vivo remains poorly understood. This project builds on our discovery to ask: how do mitochondrial metabolic states drive or protect against disease? By mapping mitochondrial function in real time within intact tissues, we aim to uncover how local shifts – like lactate accumulation – scale up to influence whole-body metabolism, with implications for diabetes, cancer, and immunity.
Quantitative characterization of tissue microenvironment
Measuring metabolites where they matter – inside living tissues.
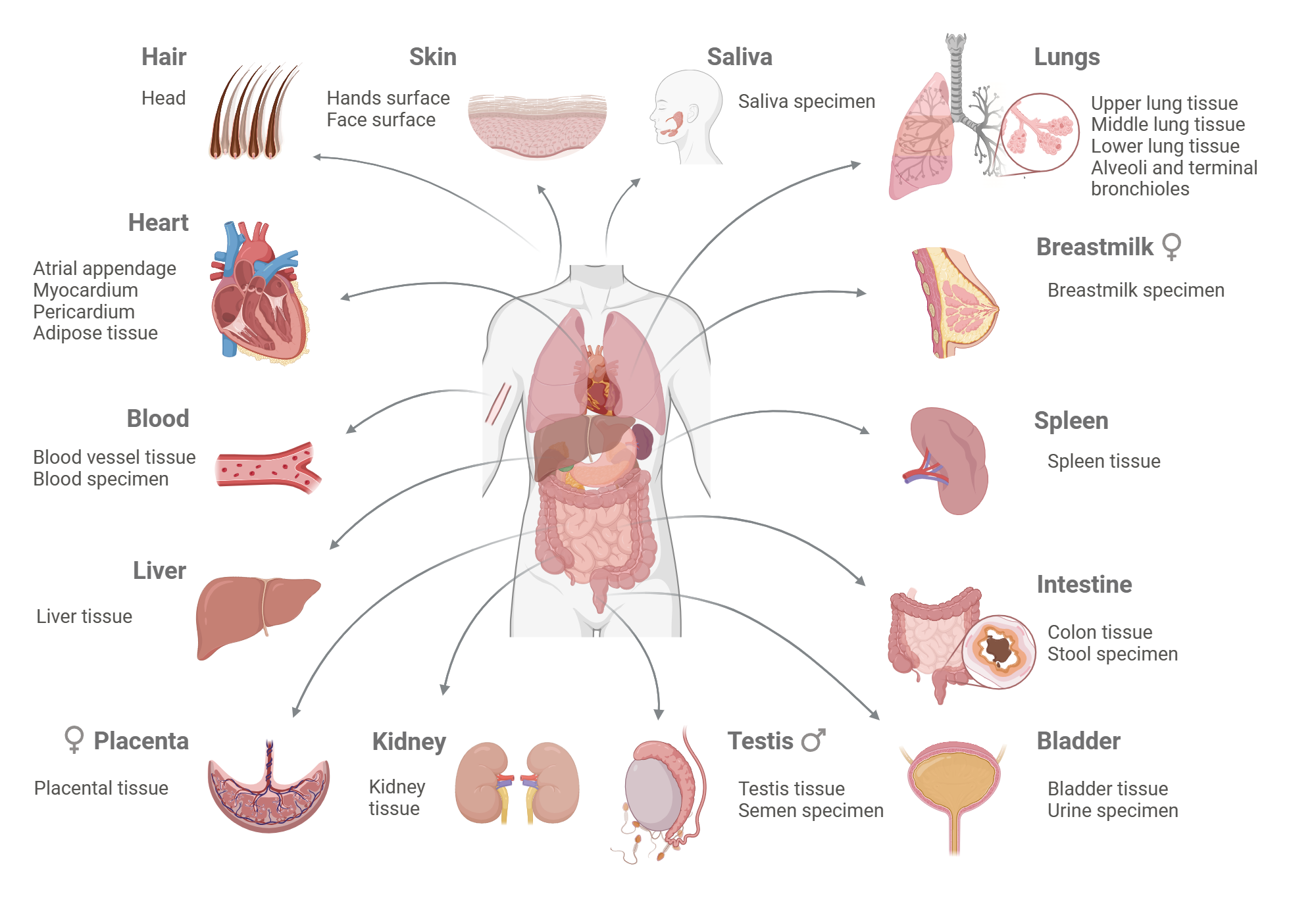
Every tissue lives in its own biochemical “neighborhood,” where local metabolite concentrations shape how cells sense nutrients, communicate, and respond to stress. Yet most of what we know about metabolism comes from blood measurements, homogenized tissue extracts, or cultured cells, which erase the fine-scale differences that define a tissue’s true microenvironment. Without absolute, quantitative data on these local metabolic landscapes, we cannot fully understand how tissues function – or fail – in health and disease. This project aims to establish new approaches for directly measuring metabolite concentrations in intact tissues under physiological conditions, providing the first quantitative maps of metabolic environments across organs. Such measurements will illuminate how tissues coordinate with one another and how disruptions in local environments drive systemic dysfunction, offering a new window into metabolic disease, cancer, and beyond.
Leverage insights from extreme physiology
Mining evolution’s solutions for resilience and repair.
Some animals thrive under conditions that would be devastating to humans – tolerating prolonged fasting, extreme cold, or dramatic tissue injury without succumbing to metabolic failure. These examples of “extreme physiology” reveal strategies that evolution has already solved for resilience, repair, and survival. Yet the molecular and metabolic logic behind these adaptations remains largely unexplored. By studying organisms and humans who naturally push the boundaries of physiology, this project seeks to uncover protective metabolic programs that can be translated into new therapeutic concepts. From tissue regeneration to metabolic disease resistance, insights from extreme biology promise to illuminate what is possible for human health.
Analytical Core Services
We offer tailored LC/MS metabolomics platforms – closely collaborating on experimental design, sample prep, and data analysis – supporting both our own research and external collaborators.
